
Sodium Chloride NaCl
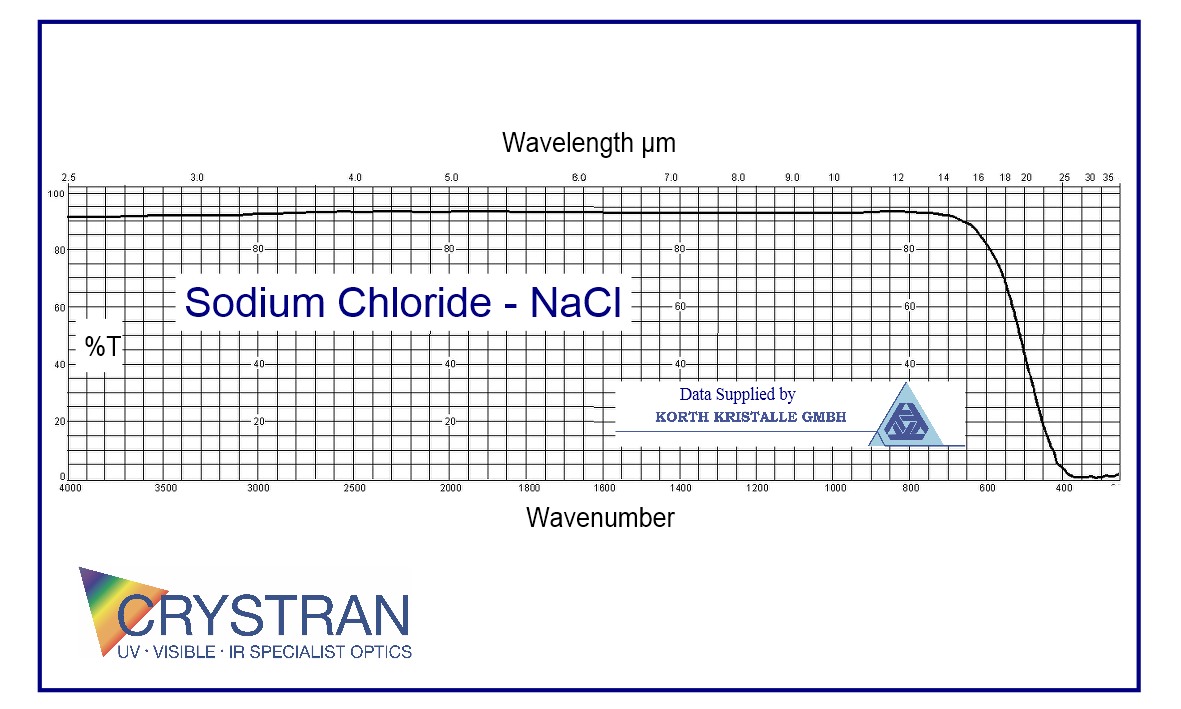
Transmission Range
0.2 to 15 μm
Refractive Index
1.49065 at 10.6 μm
Reflection Loss
7.5% at 10.6 μm (2 surfaces)
Absorption Coefficient
7 x 10-6 cm-1 @ 10.6 μm
Reststrahlen Peak
50.1 μm
dn/dT
-40.83 x 10-6 /°C
dn/dμ = 0
n/a
Density
2.17 g/cc
Melting Point
801°C
Thermal Conductivity
6.5 W m-1 K-1 @ 273 K
Thermal Expansion
44 x 10-6 K-1
Hardness
Knoop 18.2 in <100> with 200g indenter
Specific Heat Capacity
854 J Kg-1 K-1
Dielectric Constant
5.9 at 1 MHz
Youngs Modulus (E)
39.98 GPa
Shear Modulus (G)
12.61 GPa
Bulk Modulus (K)
24.42 GPa
Elastic Coefficients
C11 = 48.5 C12 = 12.3 C44 = 12.61
Apparent Elastic Limit
3.9 MPa (560psi)
Poisson Ratio
0.252
Solubility
35.7g/100g water at 273°C
Molecular Weight
58.45
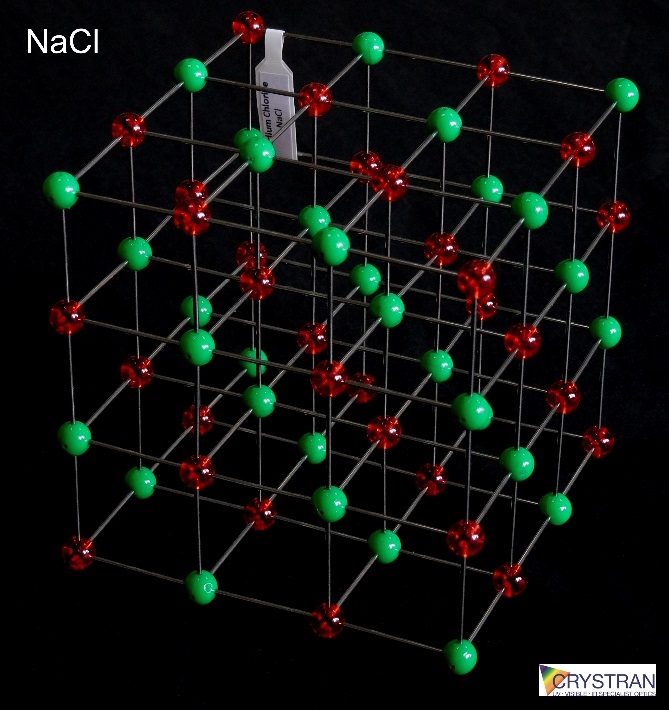
Class/Structure
Cubic FCC, NaCl, Fm3m, (#221), (100) cleavage
| µm | No |
|---|---|
| 0.589 | 1.54427 |
| 0.64 | 1.54141 |
| 0.76 | 1.53682 |
| 0.884 | 1.53395 |
| 0.972 | 1.53253 |
| 1.054 | 1.53153 |
| 1.555 | 1.53815 |
| µm | No |
|---|---|
| 2.074 | 1.52736 |
| 9.00 | 1.501 |
| 9.50 | 1.4998 |
| 10.6 | 1.49065 |
| 11.4 | 1.48476 |
| 12.5 | 1.47568 |
| 13.5 | 1.4666 |
| µm | No |
|---|---|
| 14.6 | 1.45572 |
| 16.0 | 1.4399 |
| 17.8 | 1.41649 |
| 19.8 | 1.38559 |
| 20.57 | 1.3735 |
| 22.3 | 1.3403 |
Sodium Chloride is produced in large ingots by the Kyropoulos growth method. Sodium Chloride cleaves easily. With care Sodium Chloride can be polished to a high standard under humidity controlled conditions
REFERENCES:
(1) H.H.Li, Absorption Coefficients, Int.J.Therm, V1, No. I, 1980
(2) Combes, et.al.; J.Opt. Soc. Am. V41, p215, 1951